No products in the cart.
BELTEST-IT COV-2
The BELTEST-IT COV-2 Rapid Test is an in-vitro diagnostic test for the qualitative detection of IgG and IgM antibodies to the SARS-CoV-2 virus in human serum and whole blood (venipuncture and/or fingerstick) samples collected in CLIA certified laboratories and/or by healthcare professionals at the point-of-care.
It provides healthcare professionals with a fast, convenient, and qualitative aid in the diagnosis of SARS-CoV-2 infection.*
PRODUCT INFORMATION
| Method | Immunochromatographic assay |
| Specimen Type | Human whole blood (venipuncture and/or fingerstick) or serum |
| Specimen Size | max. 50 μL |
| Time to Results | 20 minutes |
| Storage Conditions | 2°C to 30°C (35°F to 86°F) |
| Shelf Life | 12 months |
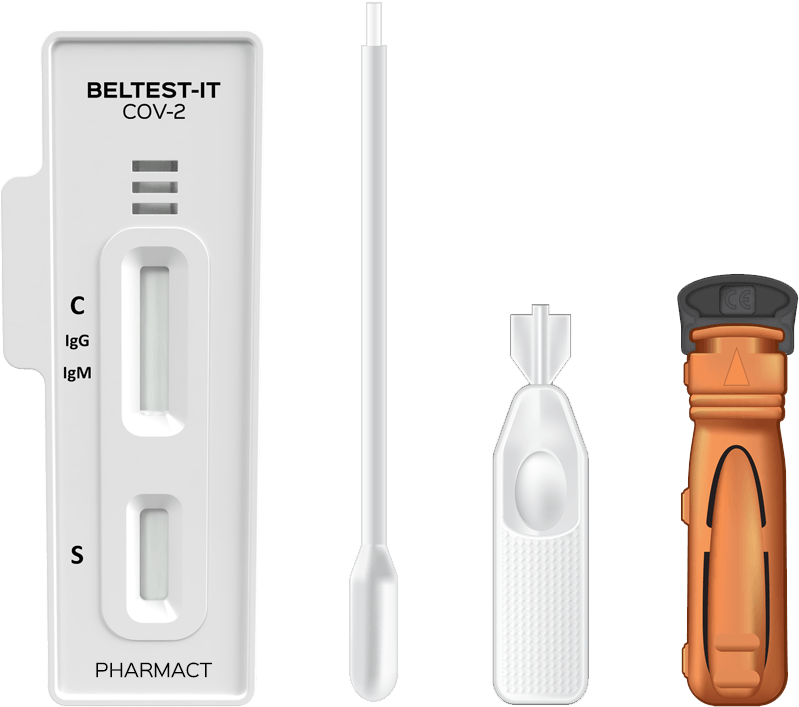
KIT CONTENTS
Test cassette
Pipette for transferring blood from the fingertip to the test cassette
Desiccant packet
Lancet
Vial containing buffer solution
Plastic bag for disposal of used test materials
Pouch with label
Instructions for use

BELTEST-IT COV-2: Instructional Video
TEST PERFORMANCE
As of June 20, 2020; a total of 1,809 tests were carried out for validation, with 643 measurements evaluated to determine the test sensitivity and 1,166 for specificity.
CROSS REACTIVITY
Pharmact tested serum samples from five HCV patients, five ANA patients, five RSV (respiratory syncytial virus) patients and six influenza (no fever) patients. All results were negative, suggesting that infection with these four diseases does not affect the specificity of the test.

